
EMMA TINSLEY
CEO
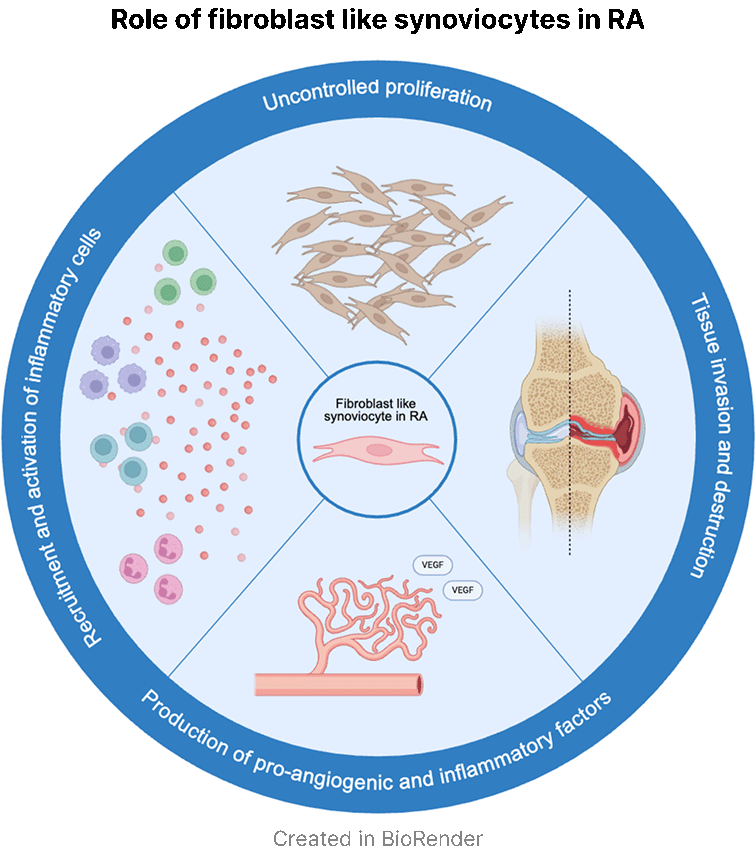
We founded Elevara Medicines to address a fundamental problem in rheumatoid arthritis (RA) where patients fail to achieve disease remission through immune targeted approaches. Proliferation and activation of fibroblast-like synoviocytes (FLS) is a central part of RA pathobiology and research over the last 20 years has shown these cells are direct contributors to the disease rather than innocent bystanders. By specifically targeting these cells, Elevara Medicines aims to break the efficacy ceiling patients experience with existing immunosuppressive drugs.
Real-world studies show that only one out of three patients with RA achieves sustained remission on TNF inhibitors (TNFi). Patients with no or poor response are switched to another targeted treatment. Up to 50% of patients, however, have a partial response: they experience significant improvement but still fail to achieve remission. For these patients the options available are to stay on treatment with partial response or switch to another mechanism of action that has no guarantee of better outcomes.
We want to change the paradigm of “ditching and switching” in RA patients with a partial but incomplete response and reduce the number of patients cycling through different MoAs.
Our drug ELV001 is a once daily small molecule targeting the proliferation and inflammatory signalling of RA FLS. The non-immunosuppressive, orthogonal mechanism of ELV001 allows us to combine it with existing immune-targeting approaches. We have preclinical and early clinical evidence to support that ELV001 synergizes with approved synthetic and biological disease-modifying anti-rheumatic drugs (DMARDs), resulting in rapid and robust efficacy (Tasaki et al., 2025).

The START-SYNERGY Trial (Synoviocyte Targeted Anti-Rheumatic Treatment) is our global Phase 2b clinical study evaluating ELV001, a first-in-class oral CDK4/6 inhibitor, as an add-on therapy for patients with rheumatoid arthritis who have an inadequate response to standard treatments such as methotrexate and TNF inhibitors.
START-SYNERGY represents a new beginning for patients living with persistent disease activity, with the goal of delivering faster relief, improved remission rates, and a safer long-term treatment option. The study will recruit patients in the United States, South Africa, Czech Republic, Poland, Bulgaria, and Serbia from December 2025.
For patients, the START-SYNERGY trial is about offering hope when current medicines are not enough. Our goal is to provide a simple oral treatment that works alongside existing therapies to reduce pain, resolve inflammation, and restore quality of life. By taking part in this study, patients may contribute to shaping a new era of care for rheumatoid arthritis.
Find out more about the START-SYNERGY trial and how it may help people living with RA.

CEO

CMO

Head of Clinical Operations

Head of Regulatory

Head of Clinical Pharmacology

Head of Biometrics
CEO
Monograph Capital
Sofinnova Partners
Forbion
Independent
London, UK – 22 October 2025 – Elevara Medicines (“Elevara”), a clinical-stage biotech developing therapies for rheumatoid arthritis (RA) and chronic inflammatory diseases, today announced the close of a $70 million Series A financing. The round was co-led by Forbion and Sofinnova Partners, with participation from founding investor, Monograph Capital.
Download Press Release